Packaging and Kitting of Pre-Filled Syringes
Packaging and Kitting of Pre-Filled Syringes
Biologics, biosimilars, vaccines and other medications administered through pre-filled syringes present unique secondary packaging challenges. First and foremost, product protection is paramount, as many parenteral pharmaceuticals are packaged in glass or rigid plastic and can be prone to breakage or cracking. Secondary packaging solutions must have the right materials so that pre-filled syringes, autoinjectors, vials and ampoules are packaged snugly… but not so tight that it risks glass or plastic cracking or breaking.
Patient safety also is a concern when discussing pre-filled syringes. Especially for self-administered kits comprising prefilled syringes, autoinjectors or other parenteral devices, child-resistant features can help prevent serious accidents. Other features, such as push-button slide trays, can increase safety and the overall administration experience through ease of use.
Manufacturing pre-filled syringes also is a broad consideration, as patient directions and needs for injectable pharmaceuticals are particularly varied, and often even customized. Far-ranging production needs mean that effective, versatile secondary packaging for pre-filled syringes and autoinjector pens must be compatible with both automated and manual manufacturing processes. From materials to manufacturing, pre-filled syringes, autoinjector pens, vials and ampoules frequently need “just right” solutions suitable for everything from limited clinical trials to full-scale commercialization.
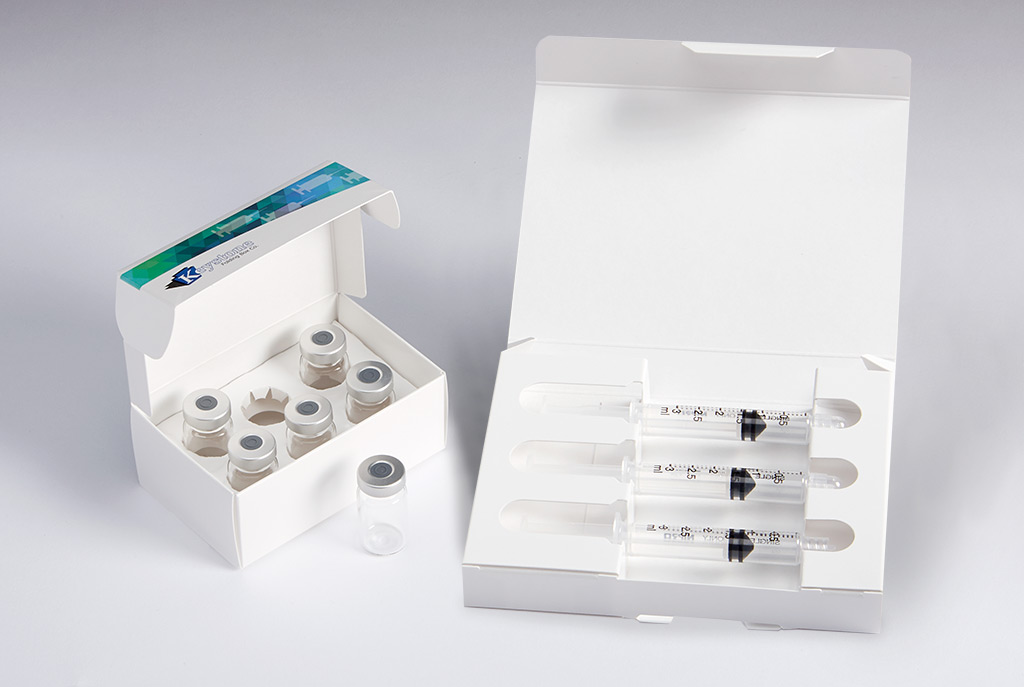
Family of Secondary Packaging Options for Pharmaceutical Prefilled Syringes, Autoinjector Pens, Vials and Ampoules
Keystone Folding Box Co. offers a comprehensive line of secondary packaging systems for injectable pharmaceutical products. Available in a variety of tailored features and in both all-paperboard and plastic-hybrid formats, solutions are available for prefilled syringes, autoinjector pens, vials and ampoules, as well as combinations of these drug delivery formats.
Keystone’s collection meets a range of needs through options such as tamper evidence and child-resistance via reclosable locking mechanisms – all while ensuring parenteral product protection throughout the pharmaceutical supply chain.
The portfolio of secondary packaging for pre-filled pharmaceutical syringes, autoinjector pens, vials and ampoules includes.
- A line of Injectable Packs suitable for pre-filled syringes or autoinjector pens, and can be designed to include ancillary items like sterile wipes
- The InjectaSlide package incorporates a thermoformed tray that slides inside a carton and into a locked position; a lock release button must be pressed to unlock and slide the tray forward for easy access to each medicine dose.
- The Vial Pack line of cartons includes designs for packaging a single vial or ampoule, or as many pharmaceutical products as a program requires.
Keystone’s secondary packaging solutions for injectables are appropriate for automated or manual production, and are ideal for both clinical trials and full-scale commercialization. Materials used are compatible with the low-temperature exposure typically found in cold-chain distribution of injectable pharmaceutical products.
Child-resistant features are available with several of the packaging solutions and, for in-home use, multi-dose packs can be designed capable of prompting patients to administer the correct dose at the proper date and time.
Keystone’s secondary packaging solutions for injectable and parenteral products exemplify our commitment to ensuring product protection and enhanced consumer experiences in every design. Injectable medicines are often high-value and delicate, and deserve protection that recognizes and respects these special circumstances.