Throughout the already rigidly regulated pharmaceuticals industry, few niches like clinical supply are as rigorously demanding of precision as clinical trials, the crucial final stage of drug development. Given the high stakes of drug development, clinical studies are understandably an environment in which drug manufacturers, materials/supplies vendors and healthcare practitioners must adhere to the highest standard of tightly controlled instructions, documentation and other requirements for clinical supplies and clinical supply use. Clinical supplies, including packaging solutions, can play a crucial role in the success or failure of clinical studies.
To aid drug developers and manufacturers, Keystone Folding Box Co. has dedicated years of time, effort and resources to developing groundbreaking clinical supply packaging solutions adopted for use by some of the most respected names in the pharmaceutical industry. This includes the area of clinical trials, the vital step after a study toward FDA approval and, ultimately, commercialization with products. Many of our clinical supply packaging solutions are naturally suited to perform as clinical supplies for sensitive clinical studies.
For starters, our packages provide pharmaceutical patients with exemplary child-resistant features necessary for clinical trial materials to meet regulatory requirements set forth by the Consumer Product Safety Commission. Keystone clinical supply solutions can be produced to F=1 child resistance – the highest designation possible for packaging.
Through its packaging solution products, Keystone also is a leader in another clinical trial must: patient compliance. Medication adherence by each patient in a clinical study with a prescribed dosing regimen is critical for medicine manufacturers to truly understand the efficacy of aspiring drugs or support treatment. Our clinical supply-ready packages can be designed to aid in medication adherence by incorporating calendarization, which helps prompt patients take the right dose at the right time.
Keystone’s clinical supplies packaging solutions also can spearhead documentation initiatives. For clinical trials where dosing data must be precisely recorded, both the Key-Pak® and Eco-Slide RX 3.0® paperboard blister pack solutions can support this task by incorporating an electronic module and printed sensor grid, which remain invisible to the patient while providing valuable clinical trial insight. This hidden technology, developed in collaboration with Information Mediary Corporation, records the time, date and location when a dose is removed from the blister package. The data can then be retrieved wirelessly which you can then use in clinical trial research.
Pharmaceutical clinical supply packaging solutions from Keystone Folding Box Co. suitable as clinical trial supplies include:
- Key-Pak is a convenient, versatile paperboard blister pack solution compatible with challenging drug platforms including friable, gel caps and liquid-filled products. The functionality of Key-Pak makes it exceptionally practical for difficult or unusual dosing situations, such as titration regimens, multi pill dosing, and more. To best assure clinical trial compliance, Key-Pak’s generous billboard space allows for large, often pictographic instructions far easier to read and follow than those printed in small font on traditional amber vials.
The Key-Pak design works with a variety of blister and lidding materials, an important factor for clinical studies. Key-Pak is compatible with either thermoformed or cold-formed blisters and can be designed for most dosing regimens.
- Made from 100% recyclable paperboard, Keystone’s Ecoslide-RX 3.0 is a child-resistant, senior-friendly compliance package that is both eco-friendly and economical to produce. Its latest iteration includes an updated re-closable locking feature designed to offer consumers a better experience. Like Key-Pak, the Ecoslide-RX 3.0 package format also allows for large, easy-to-read type and graphics on all sides of the package for clear patient instructions and product identification.
The Ecoslide-RX 3.0 carton contains no plastic, and the internal blister requires minimal film and foil. The paperboard blister pack solution passed Consumer Product Safety Commission (CPSC) protocol testing for child-resistance and received the highest rating, F=1. In addition, the Ecoslide RX 3.0 was also described as being “easy to open” in consumer testing – a helpful feature for clinical studies.
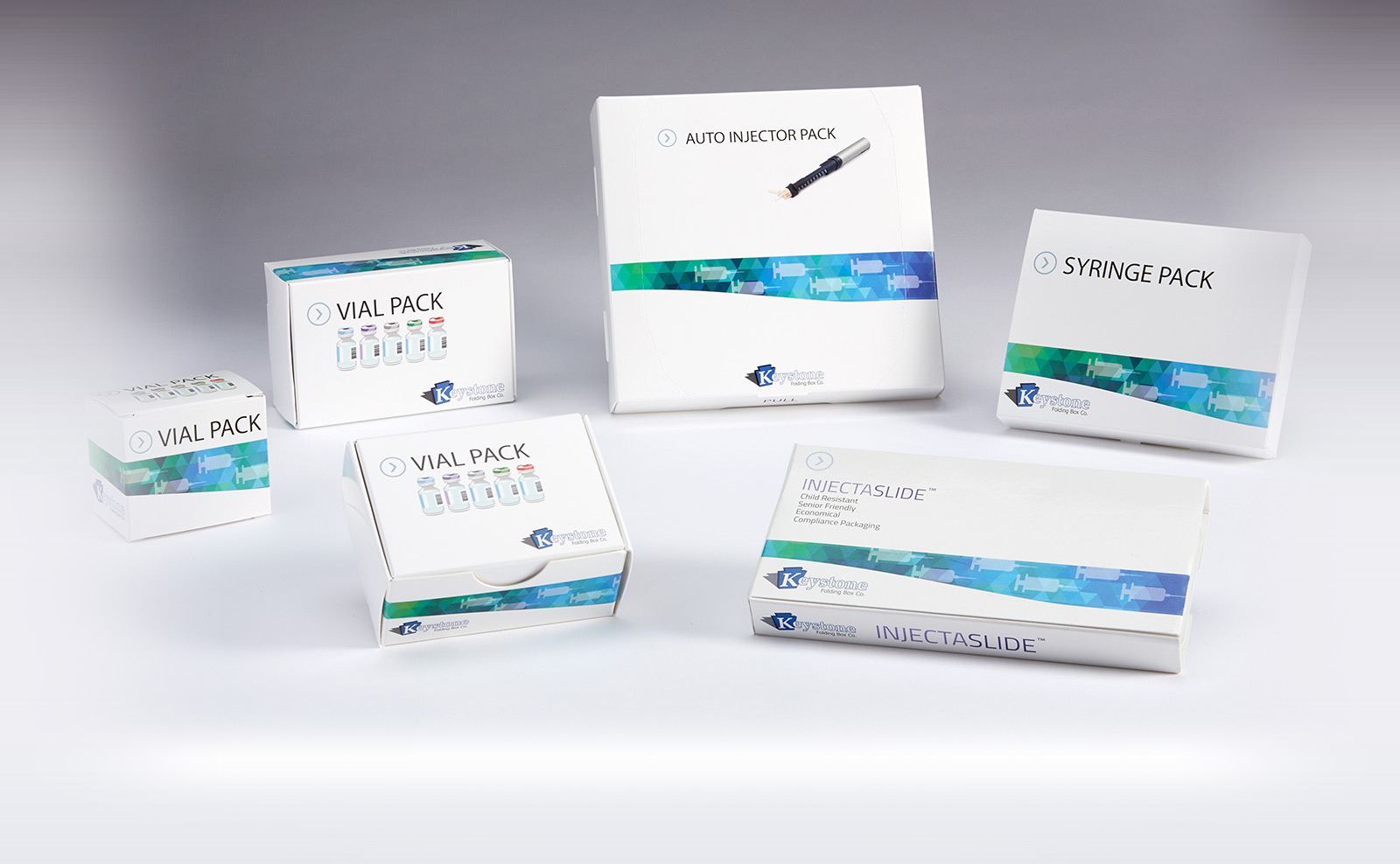
- Keystone also offers a comprehensive line of secondary packaging systems for injectable pharmaceutical products. Available in a variety of tailored features for clinical studies in either all-paperboard or plastic-hybrid formats, clinical supply solutions are available for prefilled syringes, autoinjector pens, vials and ampoules, as well as combinations of these delivery formats for drugs. Keystone’s clinical supply collection meets a range of needs through options such as tamper evidence and child-resistance via reclosable locking mechanisms – all while ensuring parenteral product protection throughout the pharmaceutical supply chain.
- A line of Injectable Packs suitable for prefilled syringes or autoinjector pens, and can be designed to include ancillary items like sterile wipes
- The InjectaSlide package incorporates a thermoformed tray that slides inside a carton and into a locked position; a lock release button must be pressed to unlock and slide the tray forward for easy access to each dose.
- The Vial Pack line of cartons includes designs for packaging a single vial or ampoule, or as many products as a program requires.