Opioid Blister Pack
Opioid Blister Pack
Drug overdoses claimed 68,000 American lives in 2018, and 72,000 the year before. The majority of these drug deaths were from opioids and heroin – which many patients turned to after initially becoming addicted to opioid painkillers.
This simply has to stop. Rare are the opportunities for pharmaceutical packaging to play such a pivotal role in widespread consumer and patient safety. But that’s exactly where we find ourselves now, and Keystone Folding Box Co. wants to be an active participant in helping solve the opioid tragedy, which has become one of the defining public health crises of modern times. One more drug death is one drug death too many.
For the past few years, the U.S. Food and Drug Administration (FDA) has been focusing on regulatory actions that decrease unnecessary exposure to prescription opioids as a way of preventing new addiction. Congress responded with the Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act – better known as the SUPPORT Act. The law is intended to help advance efforts to reduce unnecessary exposure to opioids and potentially lower the rate of new drug addiction cases.
The FDA knows that, for many people addicted to opioids, their first opioid exposure was a clinical (prescription) opioid. The FDA also has learned that patients are often prescribed significantly more opioid pills than they actually use following surgical procedures or other acute pain conditions for which opioids are prescribed. This excess supply of opioids provides opportunities for drug misuse, abuse, overdose and development of addiction, as well as the potential for these leftover pills to end up in the hands of a child, friend or relative for whom they aren’t intended.
Realizing this, FDA has determined that a significant aspect of combatting the opioid crisis must focus on encouraging “right size” drug prescribing of opioid pain medication as well as reducing the number of people unnecessarily exposed to opioids, while ensuring appropriate access to address the medical needs of patients experiencing pain severe enough to warrant treatment with opioids.
The SUPPORT Act allows the FDA to require special packaging for such drugs, including customizable fixed-quantity blister packaging, for opioids and other drugs that pose a risk of abuse or overdose. The FDA is currently seeking feedback on potential use of this new authority to require that certain immediate-release opioid analgesics be made available in fixed-quantity, unit-of-use blister packaging.
One of the biggest problems spurring the opioid crisis also is one of the simplest ones: patients are receiving too many pills. According to published studies cited by the FDA, most acute care patients used significantly fewer pills than they were prescribed for many common minimally or less-invasive surgical procedures, as well as some common acute pain conditions. Specifically, most of these patients appeared to use opioids for only one to three days following surgery and took 15 or fewer pills. Crucially, patients also reported that they usually retained unused pills in unsecure locations, providing opportunities for later drug misuse, abuse, accidental poisoning, overdose and development of addiction.
A solution to this issue also is simple: blister packaging with fixed-quantity, often diminished pill counts that do a far better job controlling what should be a tightly controlled substance.
FDA data suggests that if 5-, 10-, or 15-count unit-of-use blister package configurations of certain commonly prescribed, immediate-release opioid pain medications were made available, these options could meet the drug needs of many patients experiencing acute pain following a minimally-invasive medical procedure or other conditions commonly treated with opioid pain medications. The availability of these new packaging configurations could help prescribers to more carefully consider the amount of opioid pain medication they prescribe.
These fixed-quantity unit-of-use blister packages could even become the default option for many common acute pain conditions for which opioid pain medications are commonly prescribed, where the evidence shows that shorter durations are clinically appropriate.
The FDA opinion is clear: reducing the amount of unnecessary opioid pain medication prescribed will lead to fewer pills left in medicine cabinets that could be inappropriately accessed by family members or visitors, including children, and could potentially lower the rate of new opioid drug addiction and death.
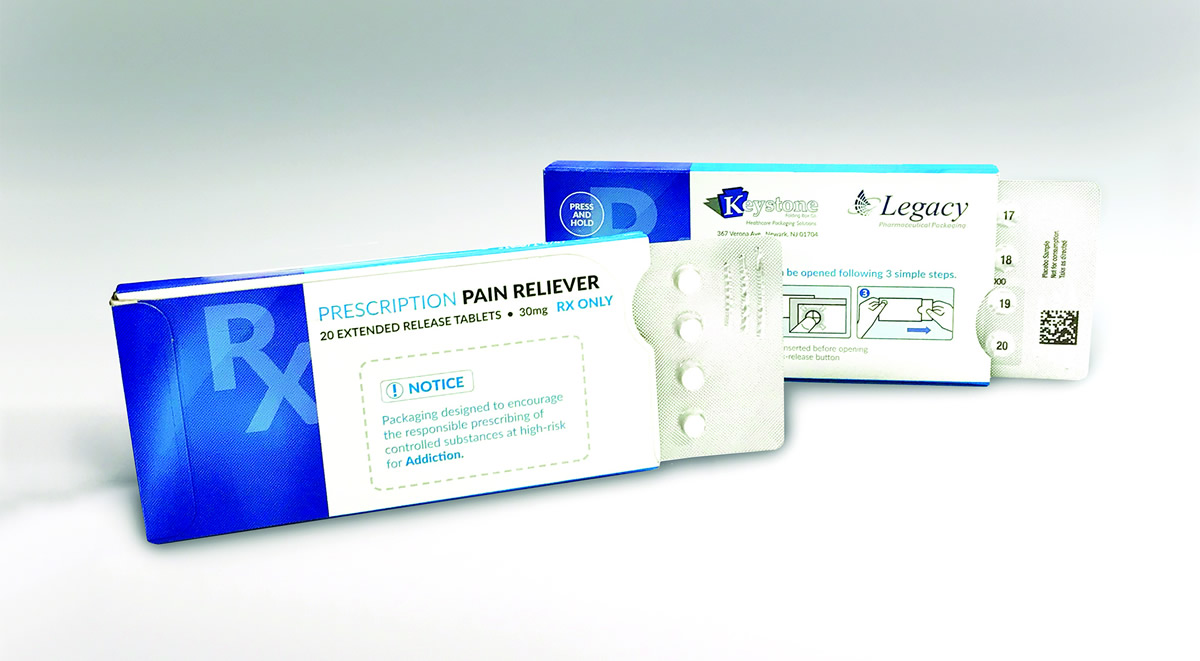
Our Solution: Ecoslide-RX for Opioid Painkillers
Keystone Folding Box Co. has developed a modified, fixed-quantity version of its Ecoslide-RX prescription blister package to address the nation’s ongoing opioid epidemic. The updated Ecoslide-RX package is designed to limit dosages for common acute pain conditions in an effort to curb overdoses while providing added benefits for safe dispensing.
The fixed quantity, typically lower dose count Ecoslide-RX for acute care was introduced in response to efforts by the FDA and the Institute for Safe Medication Practices (ISMP) to implement product packaging changes that deter opioid abuse. In an effort to combat dependency and overdoses, the design of the Ecoslide-RX allows doctors to prescribe smaller quantities of opioid medication for common acute pain conditions via limited days of dosing presented in blister packs. The fixed-quantity blister packaging also provides numerical identification of each dose, eliminating the possibility of pill-count errors.
Major benefits of Keystone’s original Ecoslide-RX package also are incorporated. The pack includes a re-closable locking feature offering consumers a safe yet streamlined experience. Child-resistant (F=1) yet senior-friendly, Ecoslide-RX is also versatile by design, meaning it can be adjusted to accommodate patients with dexterity issues. Additionally, the fixed-quantity blister pack’s broad format allows for pictographs and larger texts on all sides for easier patient instruction and accessibility.
Per its name, Ecoslide-RX also is eco-friendly: Comprised of 100% recyclable paperboard, the packaging separates easily from its internal blister for easy recycling.
Ecoslide-RX can help revolutionize the manner in which controlled substances are dispensed. It represents a forward-thinking solution that supports the pharmaceutical industry’s goal of combatting our opioid epidemic.